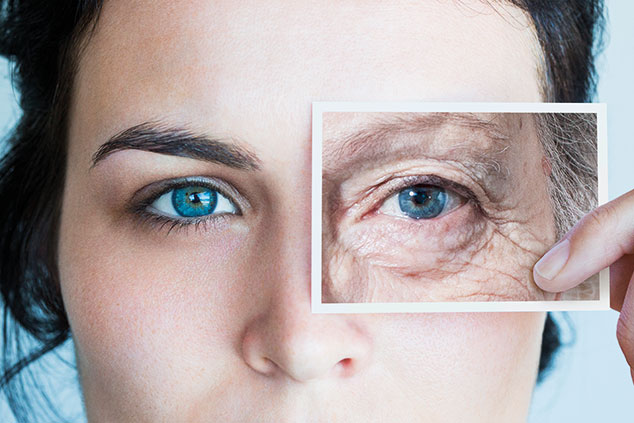
Will it be possible to turn back time?
Alas, no. The arrow of time remains implacably unidirectional. We are born, we live, and then we die. That isn’t going to change. But what is changing, according to some biologists, is that the science of anti-ageing, and anti-ageing medicine, is now a serious one – and may be on the verge of producing world-changing results. That doesn’t mean that scientists believe that the human lifespan is going to increase much from its current maximum of around 115 years. The idea of meaningful “life extension” remains a fringe pursuit among biogerontologists (scientists specialising in the biological processes of ageing). But what many believe is absolutely realistic is that within a few years, there will be drugs available that could slow down the processes associated with ageing – and boost the “healthspan” for vast numbers of us.
What is “healthspan”?
It’s the word often used in this field to denote the number of years in which an individual remains in good health, free from the common diseases associated with old age. The aim of anti-ageing research isn’t primarily to get us living longer, but to keep us healthier for as long as possible before we die: the “compression of morbidity”. Globally, life expectancy at birth has doubled over the past 150 years – to an average of 85 years in 2015. That’s great news, but it means a higher proportion of our lives (an average of 20%) is spent in “late life morbidity”– a polite way of describing the daily battle against an ever-growing burden of chronic diseases. That’s not only devastating for individuals in old age, but it’s also unsustainable in terms of the medical costs on society as a whole.
Why do we get ill as we get old?
From a biological point of view, ageing is “essentially the progressive loss of the body’s ability to repair itself, which is an unfortunate product of evolution” and natural selection, explains Graham Lawton in a recent overview of the subject for New Scientist. As our repair processes fail, damage proliferates. “Organs and tissues clog up with clumps of protein and other detritus. Genetic mutations accumulate. Chromosomes start to unravel.” Some cells become cancerous, others turn into zombies (these are called “senescent” cells). At the same time, the powerhouses of our cells, mitochondria, fall into disrepair; immune defences weaken; and low-level inflammation begins to creep through the body.
So is ageing itself an illness?
That’s increasingly up for debate. Plenty of academics (and others) would balk at the idea. Ageing is natural and inevitable, so pathologising it smacks of narcissistic denial – and perhaps economic opportunism. Yet others disagree, arguing that classing ageing as a disease would encourage much-needed investment into the field by drugs firms. The single biggest risk-factor in so many conditions (stiffer joints, thinner bones, heart failure, cancer, stroke and dementia) is old age itself. “So if we accept the notion of ageing as a disease, we would be striking at the roots of the tree of infirmity, rather than at its individual branches – the present focus of geriatrics”, says Sue Armstrong in the Financial Times. The professor of the biology at UCL, David Gems, thinks ageing should be considered a disease. In his field, genetics, scientists have discovered genes linked to ageing, and have manipulated them to extend the lifespans of model organisms such as worms. The upshot, says Gems, is that “ageing is not fixed; it can be altered”.
How can it be altered?
The focus of current efforts to do so is the study of the senescent cells – cells that have stopped replicating themselves, and instead begun to accumulate and secrete an array of molecules that promote the tissue degradation associated with ageing. Several US firms – such as Unity Biotechnology (founded by Judith Campisi, a pioneering academic expert on cellular senescence), Cleara Biotech and Senolytic Therapeutics – have developing a class of drugs called “senolytics”, which can seek out and destroy worn out cells. And scientists have also found ways of rejuvenating them, so that they can resume normal function. James Peyer, of the biotech incubator Apollo Ventures, estimates that these drugs – some of them already in clinical trials – will be in clinical use within six years.
What are the socio-economic implications of all this?
If large numbers of humans really do start to live productive, healthy lives into their 70s and 80s, the effects on societies – such as more multigenerational households and a more age-diverse workforce – are likely to be profound, suggests Camilla Cavendish in the Financial Times. The prevailing narrative is that rising numbers of elderly people will “drag down GDP and hold governments to ransom” for an ever-bigger share of welfare spending. And indeed, if people continue to retire only three-quarters of the way through their lives, the maths of mass welfare won’t stack up for long.
So what happens then?
What’s likely to happen is that older-but-still-healthy people who remain “middle-aged” into their 70s will need work for longer, phase their retirements, or even “unretire” if necessary – and the attitudes of ageist employers will need to change. For example, reckons Cavendish, older people make especially good “mentors, teachers and social workers. When there are so many social problems to fix, why don’t we put the two together” in a national programme directing the skills of elders to where they’re needed. In short, a healthier old age is likely to mean a busier and more socially-connected old age. Could it really happen? We may have only a few years to wait to find out.