Recent advances in the field of gene editing open up a multitude of exciting medical possibilities, says Simon Wilson. But the moral implications should be carefully monitored.
What’s happened?
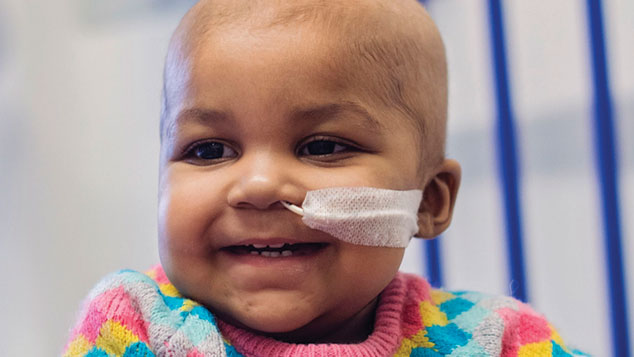
For the first time, a person’s life has been saved by a medical-scientific technique known as “gene editing” – a potentially historic breakthrough in the field of gene therapy. Layla Richards, a one-year-old girl from Enfield, was born in June 2014. At 14 weeks she was diagnosed with acute lymphoblastic leukaemia (ALL), a disease in which cancerous stem cells in the bone marrow release vast numbers of immature immune cells into the blood.
Doctors at Great Ormond Street described her condition as “one of the most aggressive cases we have ever seen”. The standard treatment – several rounds of chemotherapy and a bone marrow transplant – was unsuccessful. On the eve of her first birthday, Layla’s parents were told the only option was palliative care until she inevitably passed away.
Yet she has survived?
Layla’s specialists got in touch with consultant immunologist Waseem Qasim, professor of cell and gene therapy at University College London, who has been working with the biotech firm Cellectis on a new, untested form of gene therapy to treat cancer. The idea is to remove immune cells, genetically engineer them to attack cancerous cells, and place them back in the body. Layla’s doctors used genetically engineered immune cells from a donor – an infusion of one millilitre in a procedure lasting just ten minutes.
Within a fortnight, a rash developed indicating a positive immune response; two months later, Layla was well enough for doctors to replace the bone marrow affected by the treatment; and a month after that, she was back at home. It is too soon to say that she has been cured; that won’t be known for a year or two. But there is no sign of the cancer returning, and other patients are already being treated in the same way.
Why is this so significant?
The kind of gene editing involved could have the potential to tackle a huge range of diseases and inherited disorders, from the well known to the vanishingly rare. In almost every cell in a human body there are two copies of the human genome, one from the mother and one from the father. Each genome contains about 20,000 genes, each of which has the recipe for a specific protein in the form of a sequence of chemical “letters”.
To date, reports The Economist, medicine has recognised about 6,000 diseases which can be traced to a problem with one or another of these genes – a “disorder in which a missing or garbled sequence of DNA leaves the body unable to make a particular protein, or causes it to be made in an abnormal form”. In theory, then, gene editing’s scope could be vast.
In what sense are genes “edited”?
Conventional gene therapy “can only be used to add genes to DNA”, explains New Scientist. But with gene editing, specific DNA sequences can be cut, and broken genome sequences fixed, using “molecular scissors” – techniques which introduce mutations that disable a particular gene. The technique used in Richards’ case is known as TALEN (“transcription activator-like effector nuclease”). Another technique, older but more cumbersome, is “zinc fingers”. But the technique generating the most excitement – as it is thought to be easier to handle and more versatile – is CRISPR (“clustered, regularly interspaced short palindromic repeats”).
What are the ethical implications of gene editing?
Gene editing has potential to be used in ethically dubious ways, such as creating “designer babies”. This year, a research team in China revealed they had excised the gene for beta thalassaemia, an inherited blood disorder, from a non-viable human embryo. Even although this was a non-viable embryo, the research was so controversial that both Nature and Science declined to publish it, and a group of (mostly US) biologists called for a moratorium. But the UK’s five main medical research bodies have issued a statement supporting careful use of the technique.
What are the commercial implications?
Potentially vast – there has been a flurry of commercial activity and investment recently. In August, Editas Medicine, developing a system known as CRISPR-Cas9, raised $120m from investors including Bill Gates and Google’s venture capital arm. In April, a rival firm, CRISPR Therapeutics, raised $64m from investors including Celgene.
The promising signs for Ucart19 (the name of the cell infusion in the Richards case) are good news for Servier, the biggest French drugmaker, which has the rights to exploit the product, and for Pfizer, which has a partnership with Cellectis on similar experimental therapies. AstraZeneca is working on applications for the research, and Novartis has formed a joint-venture start-up, Intellia, with Caribou, a firm founded by Dr Jennifer Doudna, one of the pioneers of CRISPR techniques.
How does CRISPR work?
The technique is based on a trick borrowed from the bacterial immune system, explains Anjana Ahuja in the Financial Times. When invaded by a virus, bacteria deploy their own “molecular scissors” – a DNA-cutting enzyme that chops up the invader. “Pair the scissors with a guiding molecule capable of directing the blades to a specific point, and you are ready to edit a gene.”
What makes CRISPR especially exciting is that it can be used to introduce, or remove, a number of genes at the same time. That is significant because most disorders are not caused by a single gene going wrong; being able to edit many different genes in a human cell line (or indeed in a plant, or non-human animal) opens up a multitude of possibilities.